Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Yuji; Yoshimitsu, Ryo*; Nishihama, Shohei*; Shimbori, Yuma*; Shiroishi, Hidenobu*
no journal, ,
no abstracts in English
Motokawa, Ryuhei
no journal, ,
no abstracts in English
Suzuki, Hideya; Tsubata, Yasuhiro; Matsumura, Tatsuro
no journal, ,
Alkyl diamide amine (ADAAM) was investigated for the separation of MA(III). ADAAM is a multidentate ligand comprising one soft N-donor atom and two hard O-donor atoms as part of its central frame. This tridentate set of donor atoms provide selective binding for Am(III) and Cm(III) in highly acidic media and yield separation factors of up to 5.5. A continuous liquid-liquid extraction and stripping test was performed using ADAAM(EH) in n-dodecane as the extractant in a multistage countercurrent mixer-settler extractor. Separation of Am(III) and Cm(III) in very high yield was demonstrated.
Murakami, Sena; Sasaki, Yuji; Matsumiya, Masahiko*
no journal, ,
no abstracts in English
Matsumura, Tatsuro; Ban, Yasutoshi; Suzuki, Hideya; Tsubata, Yasuhiro; Toigawa, Tomohiro; Tsutsui, Nao; Hotoku, Shinobu; Suzuki, Asuka
no journal, ,
no abstracts in English
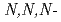 trimethylglycine
trimethylglycineSuzuki, Tomoya*; Narita, Hirokazu*; Ogata, Takeshi*; Suzuki, Hideya; Matsumura, Tatsuro
no journal, ,
no abstracts in English
Shimojo, Kojiro; Sugita, Tsuyoshi; Yabe, Makoto*; Okamura, Hiroyuki; Ohashi, Akira*; Naganawa, Hirochika
no journal, ,
Solvent extraction is an effective method for the separation and purification of metal ions. In previous study, we developed dioctyldiglycolamic acid extractant (DODGAA) with an amide group and a carboxy group connected by an ether chain, and reported DODGAA performs better than commercial extractants. In this study, we have synthesized a novel acidic diamide-type extractant (TONTADA) with two amide groups and a carboxy group connected by a nitrogen donor atom and have comprehensively investigated the extraction of 56 metal ions. It was found that the novel extractant TONTADA provides excellent extraction performance and separation ability for various rare metal ions such as Sc(III), Cu(II), Ni(II), Co(II), In(III), Ga(III), Hg(II), platinum-group metal, Hf(IV), V(V), Mo, W(VI), and Re(VII) compared with conventional extractants and DODGAA.
Sugita, Tsuyoshi; Okamura, Hiroyuki; Naganawa, Hirochika; Shimojo, Kojiro
no journal, ,
Since the solvent extraction efficiency depends on the performance of extractants, the development of high ability extractants has been desired. In this study, a novel organophosphorus extractant having a tridentate structure (DOAOBPA) was synthesized, and its extraction performance was evaluated by investigating the extraction of 56 metal ions. As a result, DOAOBPA was extremely higher than that of conventional extractants for all metal ions tested in this study. In addition, DOAOBPA can be selective separation of Pb from divalent metal ions due to the tridentate structure of DOAOBPA.
 N
N anions in the ionic liquid extraction of europium(III) chelates
anions in the ionic liquid extraction of europium(III) chelatesOkamura, Hiroyuki; Aoyagi, Noboru; Shimojo, Kojiro; Naganawa, Hirochika; Imura, Hisanori*
no journal, ,
It has been found that the extractability for neutral complexes in ionic liquid (IL) extraction systems was enhanced compared with that in organic solvent systems. However, the solvation state of the neutral metal chelates in ILs has seldom been studied. In this study, the distribution constant of neutral tris(2-thenoyltrifluoroacetonato)europium(III) (Eu(tta) ) chelate in 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C
) chelate in 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C mim][Tf
mim][Tf N])-water system was evaluated by the regular solution theory. In addition, the solvation state of the Eu(tta)
N])-water system was evaluated by the regular solution theory. In addition, the solvation state of the Eu(tta) chelate was examined by time-resolved laser-induced fluorescence spectroscopy (TRLFS) to clarify the role of the Tf
chelate was examined by time-resolved laser-induced fluorescence spectroscopy (TRLFS) to clarify the role of the Tf N
N anions in the IL extraction. Specific solute-solvent interactions between Eu(tta)
anions in the IL extraction. Specific solute-solvent interactions between Eu(tta) and [C
and [C mim][Tf
mim][Tf N] were revealed from the analysis of the distribution constant of Eu(tta)
N] were revealed from the analysis of the distribution constant of Eu(tta) with the aid of the regular solution theory. Furthermore, the TRLFS study indicated the ligand exchange between water and Tf
with the aid of the regular solution theory. Furthermore, the TRLFS study indicated the ligand exchange between water and Tf N
N in Eu(tta)
in Eu(tta) in ILs and that the coordination ability of Tf
in ILs and that the coordination ability of Tf N
N to Eu(tta)
to Eu(tta) is stronger than that of water. Therefore, the hydrophobic metal chelate is formed by the coordination of Tf
is stronger than that of water. Therefore, the hydrophobic metal chelate is formed by the coordination of Tf N
N to neutral chelates, resulting in the enhancement in the extractability.
to neutral chelates, resulting in the enhancement in the extractability.
Naganawa, Hirochika; Yanase, Nobuyuki; Nagano, Tetsushi
no journal, ,
In this symposium, the experimental result of solvent extraction of Yb (one of the rare earth elements) and collection of Al O
O solid particles at the same time by using "emulsion flow" method is introduced. The emulsion flow method, that enables simultaneous removal of dissolved and solid components, simply, rapidly and efficiently, was found to be applied to purifying wastewater of automotive aqueous paints.
solid particles at the same time by using "emulsion flow" method is introduced. The emulsion flow method, that enables simultaneous removal of dissolved and solid components, simply, rapidly and efficiently, was found to be applied to purifying wastewater of automotive aqueous paints.